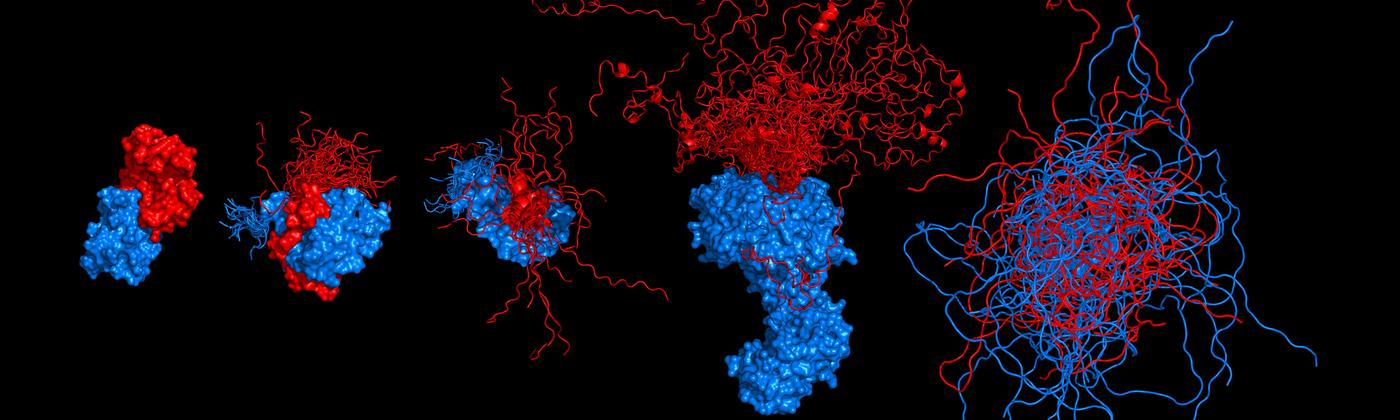
Single-molecule spectroscopy of protein folding, disorder, and dynamics
We investigate the structure, dynamics, and functions of biomolecules with single-molecule spectroscopy. In particular, we focus on systems with pronounced conformational heterogeneity that is difficult to resolve with other techniques. Examples are intrinsically disordered proteins (IDPs), protein-nucleic acid interactions, protein misfolding, or the behavior of proteins inside live cells. A key goal of our work is to reach mechanistic understanding based on quantitative physical models.
Addressing these questions requires a broad spectrum of complementary methods, and thus a multidisciplinary team of scientists from physics, chemistry, and biology who closely collaborate within the group. We use an integrative approach ranging from molecular biology and protein chemistry to a wide range of biophysical methods, single-molecule spectroscopies, and simulations. An important component of our research is the continuous development of single-molecule techniques and analysis methods for probing biological macromolecules over a wide range of conditions and timescales.